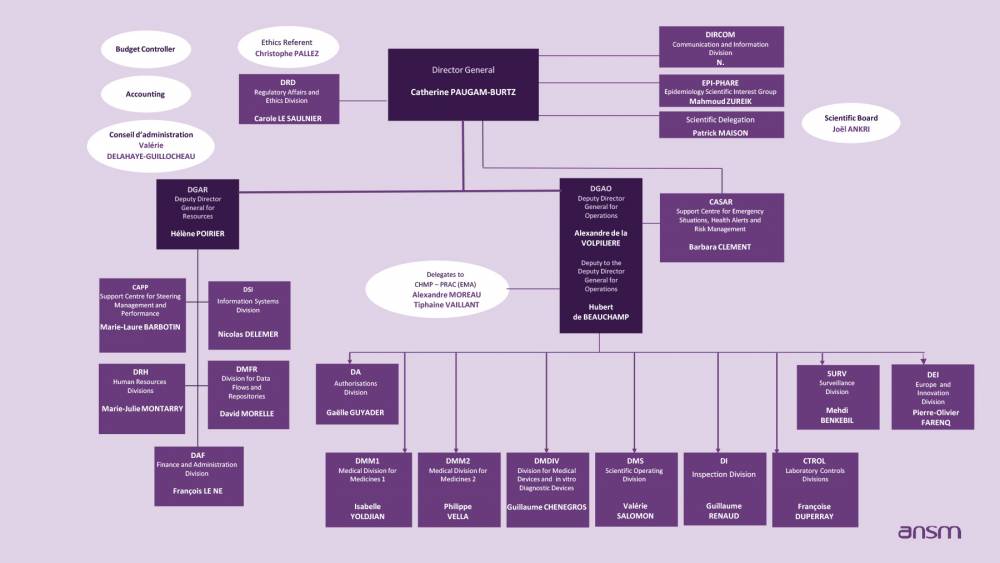
Director General’s Office
The Director General of ANSM is assisted in her duties by a Deputy Directorate-General for Operations (DGAO) and a Deputy Directorate-General for Resources (DGAR). The Regulatory Affairs and Ethics Division, the Communication and Information Division, the Scientific Delegation, and the EPI-PHARE Scientific Interest Group also report to the Director General.
We work in a collegial, cross-disciplinary and trasparent way, maintaining an ongoing dialogue with our stakeholders, in order to adopt appropriate decisions based on the opinions of several consultative committees, in which the expertise of representatives of civil society, their perspective, and their vision of the health sector allow objective and informed decision-making.
Two bodies are tasked with ANSM’s governance: a Management Board, and a Scientific Board.
Two bodies are tasked with ANSM’s governance: a Management Board, and a Scientific Board.
Overview of departments and divisions
Our divisions and departments are organised to address ANSM’s challenges which are aligned with the Objectives and Performance Contract signed with the State: a policy of openness and transparency to users, a risk management strategy as a common principle in all our decisions, improvement of our European ranking, and the stabilisation of our performance in delivering safer and more effective services in line with our publics’ expectations.Regulatory and Ethics Division
The Regulatory Affairs and Ethics Division reports to ANSM’s Director General and is responsible for the legal and regulatory security of the institution’s decisions, manages compliance with personal data protection regulations, relations with members of parliaments, and interfacing with control missions. It is involved in the drafting process for texts pertaining to the Agency’s area of competence in support of the French Ministry of Health, and in updates to legislation and regulations on a European level. It steers the financial sanction policy, manages administrative document access requests (CADA law), relations with the French Data Protection Commission (CNIL), and monitoring of external alerts. From a regulatory perspective, it coordinates prescription and dispensing conditions for medicines, and handles medicine and plasma paste import and export activities, and health product and human research qualification.It includes an Ethics and Probity Department. This department provides support, advice and legal expertise to all Agency departments in the prevention and management of potential conflict of interest scenarios. This department also carries out ethics-related information and training initiatives for all staff, or for newly appointed members of bodies.
The Director of the Regulatory Affairs and Ethics Division is ANSM’s Ethics Officer. She works closely with ANSM’s Ethics Advisor who provides external support for public service ethics issues.
The Ethics Advisor acts in an advisory and support capacity for the Director General’s Office in relation to any general queries pertaining to ethics and preventing conflicts of interest, as well as the ethics-related control environment. In addition, the Director General’s Office may request advice in cases of serious doubt associated with a staff member’s mobility status. To ensure independence and impartiality, the Ethics Advisor may not request or receive instructions from ANSM’s Director General.
- drd@ansm.sante.fr
- lanceur.alerte@ansm.sante.fr - Whistleblower/ reporting concerning a health safety alert
- exportation-medicaments@ansm.sante.fr
- importation-medicaments@ansm.sante.fr
Communication and Information Division
The Communication and Information Division reports to ANSM’s Director General, and is tasked with defining and steering the Agency’s communication and information strategy. It acts in a cross-cutting capacity internally with all staff and teams, and externally with all of our audiences. It is particularly involved in the risk management policy, the policy of openness to stakeholders, and ANSM’s aim to conduct public policies included in ANSM’s 2024-2028 Objectives and Performance Contract. The Communication and Information Division steers the Interface Committee with patient or healthcare system user associations and the Interface Committee with pharmacist representatives.Support Centre for Emergency Situations, Health Alerts and Risk Management (CASAR)
CASAR reports to the Director General and the Deputy Director General for Operations, and aims to facilitate the management of the most sensitive alerts and thus enhance the Agency's response capacity. It conducts a risk analysis including social impact, the acceptability of the situation, and risk management. It then establishes the conditions necessary for internal dialogue to implement immediate risk reduction measures.Scientific Delegation
The Delegation’s role is to provide leadership, coordination and cross-cutting strategic proposals, and to promote scientific openness. Its aim is to enhance ANSM’s scientific strategy by promoting the development of public health policies with support from the College of Advisors and the Scientific Board. It is also tasked with driving the policy of promoting public health data in concert with government policy.EPI-PHARE Scientific Interest Group
To be able to make quick, informed and independent decisions on the safety of medicines and other health products, public authorities need to be able to rely on responsive autonomous pharmaco-epidemiological and health safety expertise, capable of conducting the necessary studies.On the back of their recognised expertise and their ability to conduct routine pharmaco-epidemiological studies based on complex big data from the French National Health Database (SNDS), ANSM and the French National Health Insurance Fund (Cnam) set up the EPI-PHARE Scientific Interest Group in late December 2018.
EPI-PHARE carries out, manages and coordinates pharmaco-epidemiological studies based on complex big data from the SNDS database, in order to inform the decision-making of public authorities.
Medical Divisions
The organisation of the two medical divisions for medicines, covering areas divided by therapeutic field, has been redesigned in concert with the Authorisations Division and the Scientific Operating Division to enable increased communication with users within the scope of the appraisal of applications, and also thanks to collective facilitation of the permanent committees falling within their remit. In this way, they promote openness to stakeholders though direct links with patient associations and healthcare professional representatives.DMM1 - Medicines indicated in the treatment of: oncology, oncohaematology, haematology, nephrology, transplantation, cell therapy, transfusion, plasma-derived medicines, radio-pharmaceutics, contrast media, cardiology, vessels-thrombosis-intensive care, antidotes-stomatology, ophthalmology, Endocrinology (diabetology), gynaecology and obstetrics, urology, allergology, pneumology, ENT
DMM2 - Medicines indicated in the treatment of: psychiatry, neurology, pain control, anaesthesia-rheumatology, addiction medicines, dermatology, enzyme deficiencies, internal medicine, hepatology, gastroenterology, vaccines and anti-infectious agents, virology, emerging diseases, regulation of the flow of narcotic and psychotropic substances
- dmm2@ansm.sante.fr
- stupetpsy@ansm.sante.fr - Authorisations for narcotics and psychotropic substance (use - import - export - annual reporting - theft reporting)
MDIVD - Medical Devices and In Vitro Diagnostic Medical Devices
The primary role of the Medical Division for medical devices and in vitro diagnostic medical devices, with a remit covering all medical disciplines, is to ensure patient safety through early identification of risks and implementation of action plans to control these risks, including appropriate market surveillance initiatives. It also issues scientific and regulatory opinions and plays an active role, as France’s designated competent authority, in the various European projects for the implementation of regulations applicable to medical devices and in vitro diagnostic medical devices.- dmcdiv@ansm.sante.fr
- EC.DM-COS@ansm.sante.fr Clinical trials - Medical devices and in vitro diagnostic medical devices
- cnq.labm@ansm.sante.fr - National quality control of medical laboratory tests
- cosmetovigilance@ansm.sante.fr - Cosmetic product vigilance
Authorisations Division
With coordinated centralised management of marketing authorisation (excluding centralised procedures) and clinical trials (excluding early phases), the role of the Authorisations Division is to secure and harmonise the medicine authorisation process, particularly by introducing a risk analysis and a collective approach into the appraisal of applications and ensuring the management of priorities and deadlines, for the benefit of patients. It now acts at the sole point of contact for the healthcare sector and operators.Europe and Innovation Division
The role of the Europe and Innovation Division is to coordinate innovation, clinical research and early and compassionate access policy in concert with the Medical Divisions and the Scientific Operating Division, and implementation of the European medicine evaluation strategy. This division is also tasked with representing the Agency in the major European committees (CHMP, PRAC, CMDh, SAWP, CAT, HMA), where it promotes an integrated vision of access to innovation, in France and in Europe.- innovation@ansm.sante.fr - Innovation Service
Scientific Operating Division
Using its high-quality scientific approach, the role of the Scientific Operating Division is to safeguard the quality of products by incorporating a multidisciplinary approach into the assessment of products and appraisal of applications. With this in mind, it promotes openness to stakeholders aimed at increasing confidence in topics relating to the toxicity and pharmaceutical quality of biological and chemical products. The Division supports the French National Pharmacopoeia Authority. It “monitors the health of products”.- contactspharmacopeefrancaise@ansm.sante.fr - French Pharmacopoeia
- preparations@ansm.sante.fr - Preparations
Surveillance Division
The Surveillance Division is involved in the surveillance of health products through its cross-cutting pharmacovigilance, addiction vigilance, haemovigilance, medical device vigilance, reagent vigilance and clinical trial vigilance activities. It organises the detection signals and unknown adverse reactions, risk-based prioritisation of applications for review by the Medical Divisions, safeguarding of measures via the organisation of collective discussions internally and with vigilance networks, steering of the Medical Divisions’ vigilance activities, and database administration. These surveillance duties are also carried out proactively to identify an initial risk, even in the absence of signals. The Surveillance Division also encompasses specific expertise in the fields of pregnancy, reproduction and breastfeeding, medication error prevention and management, advertising control, and medicinal cannabis. It contributes to the vigilance data transparency policy and steers and coordinates two public health policies relating to pregnancy and misused prevention.- materiovigilance@ansm.sante.fr - Medical device vigilance
- pharmacovigilance@ansm.sante.fr - Pharmacovigilance
- reactovigilance@ansm.sante.fr - Reagent vigilance
- visapublicite@ansm.sante.fr - Advertising approval
Inspection Division
The Inspection Division monitors the quality of practices among operators (manufacturers, operators, importers, distributors, clinical trial sponsors, investigators, trial facilities, etc.), as well as the quality and safety of health products, including starting materials. It helps to define enforceable regulatory frameworks (especially good practices), on national, community and/or international levels; it manages sites (authorisations, accreditations, declarations, sanctions, etc.) and ensures that the enforceable regulatory provisions are implemented, via on-site inspections (in France or abroad) in the context of an annual or spot-check inspection programme. It manages reports relating to medicine and active substance defects, as well as reports potentially leading to an inspection for other health products, and clinical trials. Finally, it helps tackle medicine shortages, ensuring the availability of medicines of medicines of major therapeutic value and of those whose unavailability would pose a public health risk. In concert with the Agency’s other divisions, it coordinates the actions that pharmaceutical companies must take to secure patients’ access to these medicines.- insevi@ansm.sante.fr - Inspection of tests and vigilance
- BPC@ansm.sante.fr - Trials and vigilance inspection
- BPL@ansm.sante.fr - Good laboratory practices and inspection
- insmp@ansm.sante.fr - Starting materials inspection
- insbio1@ansm.sante.fr - Biological products inspection
- Inspection.PSL@ansm.sante.fr - Biological products/Labile blood products inspection
- biosecurite@ansm.sante.fr - Biological products/Microorganisms and toxins inspection
- ipplf@ansm.sante.fr - Pharmaceutical products inspection and fraud control
- fraude@ansm.sante.fr - Pharmaceutical product inspection and anti-fraud
- insmar.dm@ansm.sante.fr - Market surveillance inspection/Medical device-related sites
- insmar.div@ansm.sante.fr - Market surveillance inspection/In vitro diagnostic medical device-related sites
- insmp@ansm.sante.fr - Registration and inspection of pharmaceutical starting material manufacturing, distribution and import sites
- rupture-stock@ansm.sante.fr - Stock and supply shortages
- dvs.defauts-qualite@ansm.sante.fr - Quality defects
Quality Control Division
The Quality Control Division has two main roles: laboratory verification of the intrinsic quality of products according to a market surveillance approach, within the framework of European and international surveillance programmes alongside the European Directorate for the Quality of Medicines and Health Care (EDQM), the European Medicines Agency (EMA) and the World Health Organization (WHO), or urgently, and a batch control and release activity in respect of immunological medicines (vaccines) and blood-derived medicines prior to market release, for the French market and for the European market within the framework of procedures led by the EDQM for the network of Official Medicines Control Laboratories (OMCLs), of which ANSM is a member. As such, it is the national official laboratory.It also acts on behalf of international markets within the framework of the WHO prequalification procedure and contributes to the development of control methods and/or to the qualification of reference substances and participates in drafting reference documents in cooperation with standardisation bodies.
Data Flow and Repository Division
The Data Flow and Repository Division is an important link in the “operations” chain. It is the first point of contact for application submission and traceability. It feeds the proprietary medicine registry, the public medicines database and several operating repositories. This division controls the documentary circuit and manages the Agency’s archival policy, the compilation of historic Archives, and their transfer to the National Archives. It also manages the user reception department.- declaration.CODELPP@ansm.sante.fr - LPP code reporting
- communications.DM@ansm.sante.fr - Reporting and communication for medical devices and operators
- pgref@ansm.sante.fr - Forwarding of generic MA holder email addresses
Three resource departments
Human Resources, Administration and Finance and Information Systems – provide ANSM as a whole with all necessary human, financial and logistical resources, procedures, methods and tools to fulfil its various duties. The Accounting Office completes this organisation.- rh@ansm.sante.fr - Human Resources Division
- dpi@ansm.sante.fr - Expert declaration of interests
- ansm-factures@ansm.sante.fr - Invoices-Suppliers
- experts.missions@ansm.sante.fr - Expert business expenses