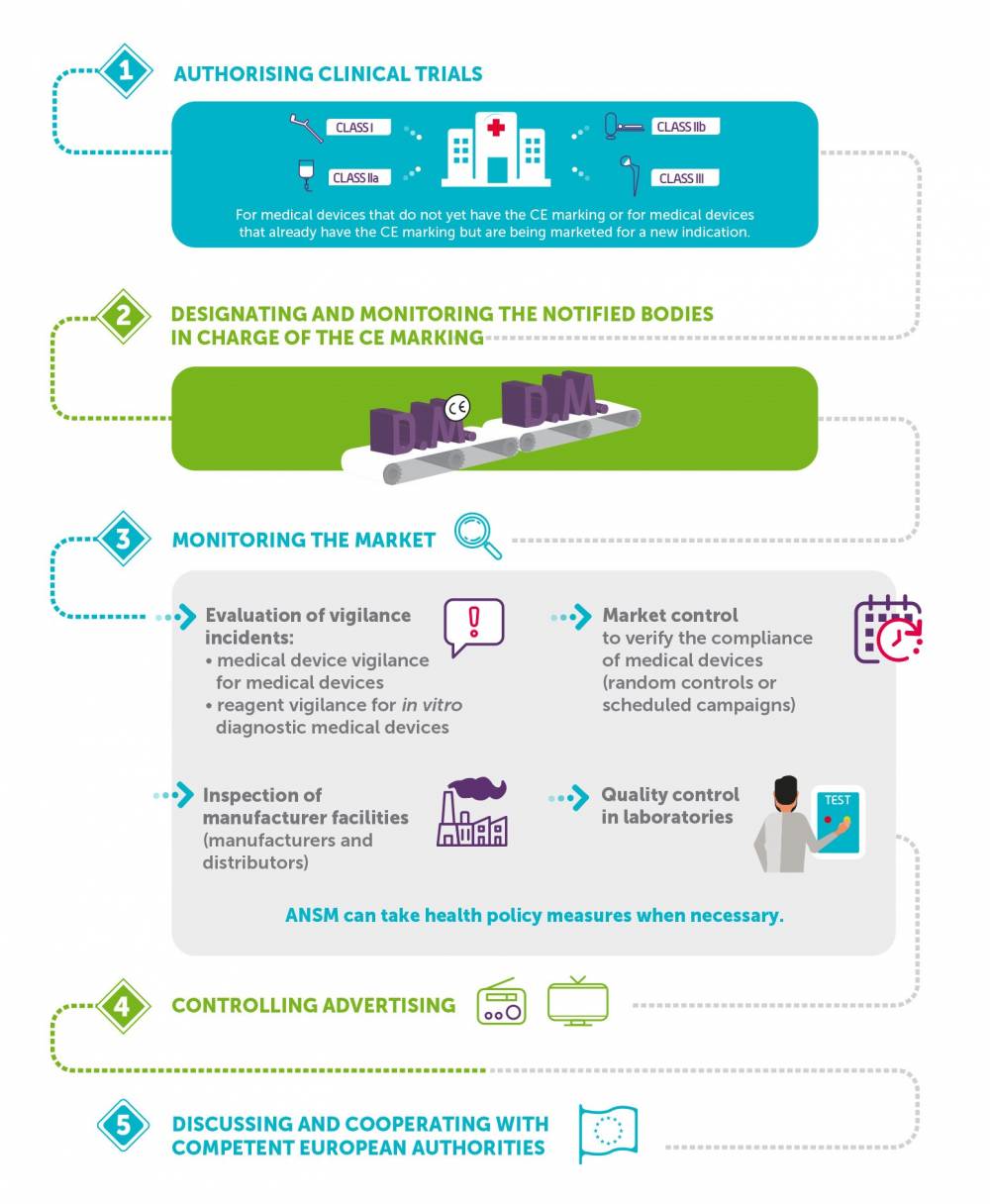
Medical devices and in vitro diagnostic medical devices (IVDMDs)
Medical devices
A medical device is any instrument, apparatus, equipment, material or product (except products of human origin), including accessories and software, that is used alone or in combination, for medical purposes in humans, and which does not achieve its principal intended action by pharmacological, immunological or metabolic means.The medical device market is extremely vast and the sector is highly innovative. The industrial network is multiple and diversified; it includes both large multinational groups and small and medium-sized enterprises.
It includes over 20,000 types of products, ranging from single-use or reusable consumables (dressings, compresses, etc.), to implants (breast prostheses, pacemakers, etc.), also including equipment (medical beds, etc.).
Different medical device classes
Medical devices are classified according to level of risk associated with their use (duration of use, exposed part of the body, inside or outside the body), and the potential risks associated with their use for public health (class I to III).These risk classes hence also express the expected medical benefit for the patient.
- Class I (lowest risk class): e.g. compresses, spectacles, crutches, etc.;
- Class IIa (moderate/modest potential risk): e.g. contact lenses, ultrasound devices, dental crowns, etc.;
- Class IIb (high/significant potential risk): e.g. condoms, lens disinfection products, etc.;
- Class III (highest risk class): e.g. breast implants, stents, hip prostheses, etc.
In vitro diagnostic medical devices
An in vitro diagnostic medical device is a product or instrument, including accessories and software, intended by its manufacturer to be used in vitro in the examination of samples of human origin (blood, urine, tissues, etc.), for the purpose of providing information, particular about an individual’s physiological or pathological state or about a congenital anomaly.Tests (COVID-19 test, pregnancy test, blood glucose assay test, etc.), also known as “reagents”, used in laboratories to conduct pathology tests particularly fall within this category.
Different in vitro diagnostic medical device categories
Regulation (EU) 2017/746 identifies several IVDMD categories.This prescriptive classification defines “lists” (A and B) including IVDMDs having a high risk for patients and for public health. Depending on whether they are included in these lists or not, the IVDMDs in question are not subject to the same conformity requirements.
The directive also makes a distinction between devices for self-diagnosis and IVDMDs for assessing performances, and IVDMDs which do not fall under List A, List B, or self-diagnosis.