Organising vigilance
The vigilance system is organised around the collection and review of adverse event reports. The purpose of this system is to identify unexpected alert signals observed during wide use of the health products as early as possible, and thereby implement the public health required to guarantee patient safety.
ANSM is responsible for five vigilance categories
ANSM also carries out clinical trial vigilance.
www.signalement-sante.gouv.fr
An online reporting portal for adverse health events, www.signalement-sante.gouv.fr, has been available since 2017 thanks to a partnership between the French Ministry of Health and healthcare agencies, in particular ANSM. This portal is designed to increase health safety vigilance while also simplifying the reporting process. It is accessible to everyone, including both healthcare professionals and users.
Pharmacovigilance
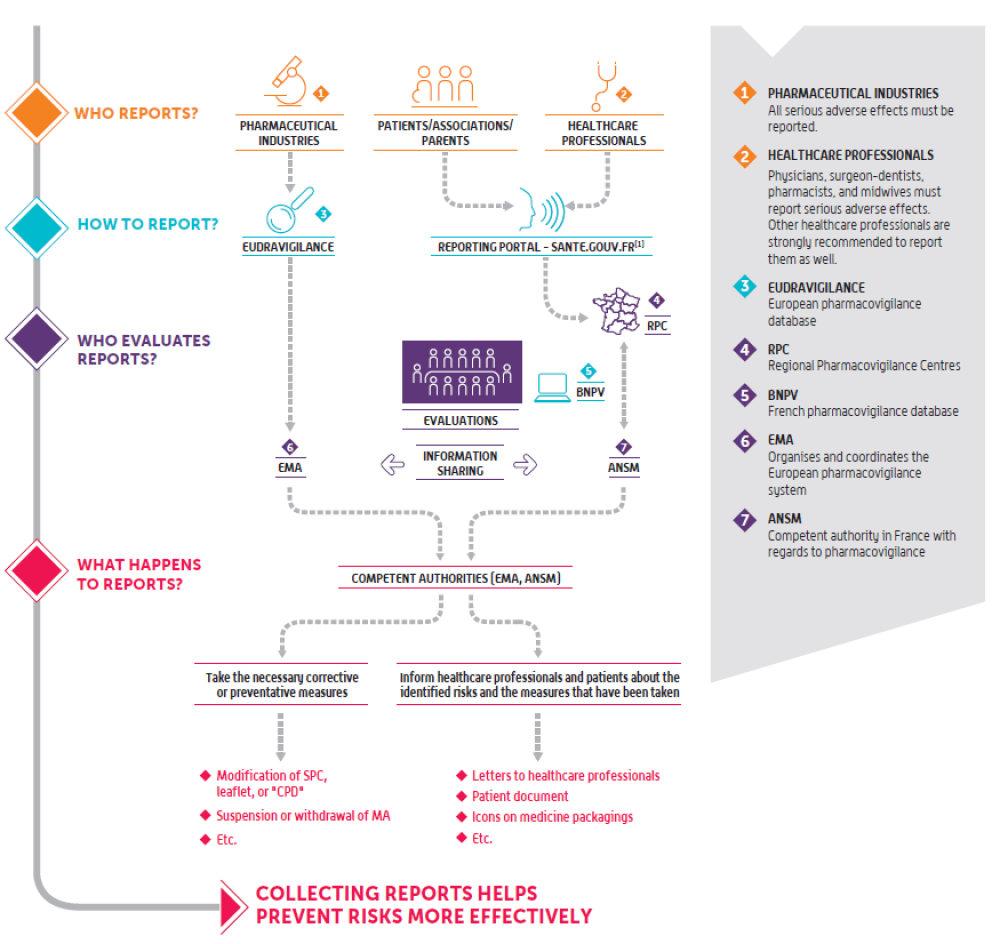
Pharmacovigilance is carried out for all medicines used by patients in France: it examines adverse reactions that occur under normal conditions of use, as well as those that arise due to medication errors, abuse, misuse, overdose, and occupational exposure.It is active at the regional level through France’s 31 regional pharmacovigilance centres (CRPV), at the national level through ANSM, and at the European level through the European Medicines Agency (EMA) and Member States.
Find out more about French pharmacovigilance
Healthcare professionals and users of the health system report adverse reactions that they believe to be related to the use of a medicine. Doctors, dentists, pharmacists and midwives have an obligation to report such reactions.
The reports are then recorded by a regional pharmacovigilance centre (CRPV) in the French national pharmacovigilance database (BNPV). This database can be accessed by all CRPV and ANSM.
The declaration is subject to a review by the CRPV, which investigates the reported case and, if necessary, discusses with the healthcare professional and/or the patient who submitted the report. It then determines the causality of the medicine with regard to the adverse reaction reported, particularly in light of the existing data, the context of use and the profile of the patient concerned.
CRPV draw ANSM’s attention to reports that constitute potential signals, which are termed “noteworthy cases”, and perform expert assessments. These assessments make it possible to confirm, dismiss, and, where necessary, characterise signals and risks.
At the same time, pharmaceutical companies are required to submit any medicine-related adverse reaction reports they record directly to the European pharmacovigilance database (EudraVigilance).
ANSM liaises with CRPV on an ongoing basis to identify any risk or change to a known risk.
It takes the reported signals, reviews them using its medical, scientific and technical expertise, and communicates with the healthcare professional and patient representatives concerned. ANSM may also enlist an CRPV to conduct additional investigations on a set of reports with a view to enhancing the characterisation of a potential or known risk.
Where appropriate, ANSM puts in place the necessary measures to prevent or reduce risks in order to ensure the safe use of medicines, in consultation with external partners.
The reports are then recorded by a regional pharmacovigilance centre (CRPV) in the French national pharmacovigilance database (BNPV). This database can be accessed by all CRPV and ANSM.
The declaration is subject to a review by the CRPV, which investigates the reported case and, if necessary, discusses with the healthcare professional and/or the patient who submitted the report. It then determines the causality of the medicine with regard to the adverse reaction reported, particularly in light of the existing data, the context of use and the profile of the patient concerned.
CRPV draw ANSM’s attention to reports that constitute potential signals, which are termed “noteworthy cases”, and perform expert assessments. These assessments make it possible to confirm, dismiss, and, where necessary, characterise signals and risks.
At the same time, pharmaceutical companies are required to submit any medicine-related adverse reaction reports they record directly to the European pharmacovigilance database (EudraVigilance).
ANSM liaises with CRPV on an ongoing basis to identify any risk or change to a known risk.
It takes the reported signals, reviews them using its medical, scientific and technical expertise, and communicates with the healthcare professional and patient representatives concerned. ANSM may also enlist an CRPV to conduct additional investigations on a set of reports with a view to enhancing the characterisation of a potential or known risk.
Where appropriate, ANSM puts in place the necessary measures to prevent or reduce risks in order to ensure the safe use of medicines, in consultation with external partners.
Find out more about France’s contribution to European and international pharmacovigilance
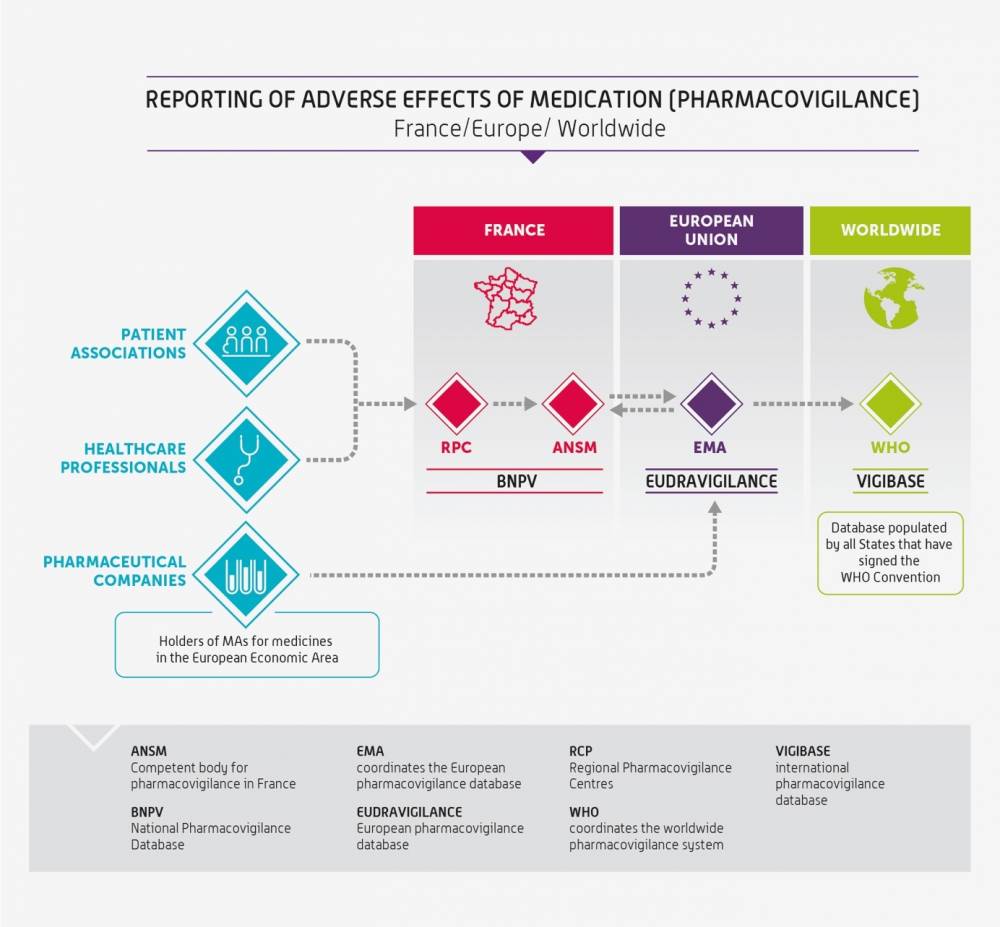
The French national pharmacovigilance system is part of a European framework. France therefore works closely with the European Medicines Agency (EMA) and the other Member States to monitor and ensure the safe use of medicines in France and the rest of the European Union.
ANSM plays an active role in European working groups, in particular the Pharmacovigilance Risk Assessment Committee (PRAC), which helps monitor and address changes to the benefit-risk ratio of medicines: referrals, signals, post-marketing safety studies, etc.
ANSM may also be requested to conduct a scientific assessment on a medicine (or class of medicines) on behalf of the European Union, within the scope of a referral procedure. This procedure is used to address a concern regarding a medicine’s safety or benefit-risk ratio. It can also be used to settle a disagreement between Member States concerning a medicine's use. Its ultimate aim is to provide a harmonised guidelines in the European Union.
In addition, ANSM and pharmaceutical companies transmit data to EudraVigilance, the European Medicines Agency (EMA) database, on a daily basis. This database is the sole collection site in Europe for all serious adverse reactions and, since November 2017, non-serious adverse reactions occurring in Europe.
At the international level, the World Health Organization established an international pharmacovigilance database in 1968: VigiBase. This is the largest and most complete database in the world. VigiBase is maintained by the Uppsala Monitoring Centre (UMC) under WHO’s mandate. More than 150 countries help collect pharmacovigilance data. France has participated in the programme since 1986. France is the 7th largest contributor, providing approximately 2,5% of the total number of adverse reaction reports received.
ANSM plays an active role in European working groups, in particular the Pharmacovigilance Risk Assessment Committee (PRAC), which helps monitor and address changes to the benefit-risk ratio of medicines: referrals, signals, post-marketing safety studies, etc.
ANSM may also be requested to conduct a scientific assessment on a medicine (or class of medicines) on behalf of the European Union, within the scope of a referral procedure. This procedure is used to address a concern regarding a medicine’s safety or benefit-risk ratio. It can also be used to settle a disagreement between Member States concerning a medicine's use. Its ultimate aim is to provide a harmonised guidelines in the European Union.
In addition, ANSM and pharmaceutical companies transmit data to EudraVigilance, the European Medicines Agency (EMA) database, on a daily basis. This database is the sole collection site in Europe for all serious adverse reactions and, since November 2017, non-serious adverse reactions occurring in Europe.
At the international level, the World Health Organization established an international pharmacovigilance database in 1968: VigiBase. This is the largest and most complete database in the world. VigiBase is maintained by the Uppsala Monitoring Centre (UMC) under WHO’s mandate. More than 150 countries help collect pharmacovigilance data. France has participated in the programme since 1986. France is the 7th largest contributor, providing approximately 2,5% of the total number of adverse reaction reports received.
Addiction vigilance
Addiction vigilance consists of monitoring, assessing, preventing and managing the risk of abuse, misuse and drug dependence associate with the consumption, for medicinal purposes or not, of any psychoactive substance (plant or product), excluding alcohol and tobacco.At the national level, addiction vigilance relies on a network of 13 Drug Dependence-Addiction Evaluation and Information Centres (CEIP-A) managed by ANSM.
Find out more about French addiction vigilance
Healthcare professionals report cases of abuse and dependence relating to medicines containing a psychoactive substance (or having a psychoactive effect) that have come to their attention to their local drug dependence-addiction evaluation and information centre (CEIP-A).
In addition, pharmaceutical companies are required to submit any medicine-related adverse reaction reports they record directly to the European pharmacovigilance database (EudraVigilance).
A significant addiction vigilance report consists of one or more cases of abuse, dependence, misuse, trafficking (gift, dealing, buying on the street, resale, forged prescription, Internet, etc.) or any other use disorder relating to a psychoactive substance, whether it is medicinal or not, except for alcohol and tobacco, reported by a CEIP-A to ANSM.
This case may have a reported clinical effect or involve one (or more) substance(s) and an identified user who may or may not be linked with a specific user, or have no reported clinical effect, such as a report of forged prescription(s), for example.
Addiction vigilance surveys
The objective of the national addiction vigilance survey is to assess the risk of abuse, dependence, misuse and improper use of a psychoactive substance, whether it is medicinal or not, with a view to confirming a potential signal, characterising a proven signal, monitoring the risk profile of a medicine, or measuring the impact of a regulatory measure.
The survey is conducted by a reporting expert, assisted by a reviewing expert, members of the CEIP-A centres, at ANSM’s request. The conduct of the survey must meet the objectives set by ANSM within the allotted time frame
Specific tools of the addiction vigilance network
The addiction vigilance network conducts annual pharmaco-epidemiological surveys to help monitor psychoactive substance use:
Cases of abuse and dependence involving a medicine must be entered in the BNPV. The BNPV may be consulted in the context of a survey or to verify a report.
In addition, pharmaceutical companies are required to submit any medicine-related adverse reaction reports they record directly to the European pharmacovigilance database (EudraVigilance).
Surveillance tools
Detecting significant reports in addictovigilanceA significant addiction vigilance report consists of one or more cases of abuse, dependence, misuse, trafficking (gift, dealing, buying on the street, resale, forged prescription, Internet, etc.) or any other use disorder relating to a psychoactive substance, whether it is medicinal or not, except for alcohol and tobacco, reported by a CEIP-A to ANSM.
This case may have a reported clinical effect or involve one (or more) substance(s) and an identified user who may or may not be linked with a specific user, or have no reported clinical effect, such as a report of forged prescription(s), for example.
Addiction vigilance surveys
The objective of the national addiction vigilance survey is to assess the risk of abuse, dependence, misuse and improper use of a psychoactive substance, whether it is medicinal or not, with a view to confirming a potential signal, characterising a proven signal, monitoring the risk profile of a medicine, or measuring the impact of a regulatory measure.
The survey is conducted by a reporting expert, assisted by a reviewing expert, members of the CEIP-A centres, at ANSM’s request. The conduct of the survey must meet the objectives set by ANSM within the allotted time frame
Specific tools of the addiction vigilance network
The addiction vigilance network conducts annual pharmaco-epidemiological surveys to help monitor psychoactive substance use:
- in centres specialised in drug user care (e.g OPPIDUM: observation of illegal or misused psychotropic substances),
- in community pharmacies (e.g. OSIAP: suspect prescriptions, indicators of possible abuse, ASOS: narcotic analgesics and secure prescriptions)
- with toxicology experts (e.g. DRAMES: deaths related to medicine and substance abuse, DTA: drug-poisoning deaths involving analgesics, Chemical dependence).
Cases of abuse and dependence involving a medicine must be entered in the BNPV. The BNPV may be consulted in the context of a survey or to verify a report.
Find out more about European and international addiction vigilance
What does ANSM do?
What does ANSM do?
Participation in the European addiction vigilance system
ANSM participates in the information exchange and risk assessment system. This system operates on a European scale and assesses psychoactive substances of low therapeutic benefit. The system is also used to trigger alerts on the risks associated with these substances, which are disseminated in all Member States. The system uses a network structure, with 15 information centres located in the different Member States. These centres collect and analyse data on drugs.What does ANSM do?
- Sends to EUDA (European Union Drugs Agency) the evaluation reports produced by the CEIP-A.
- It reports cases of abuse associated with the consumption of proprietary medicines to the European Medicines Evaluation Agency (EMEA).
- It assesses the risks of abuse and dependence generated by the consumption of proprietary medicines within the framework of a European registration procedure.
Participation in the international addiction vigilance system
WHO and the UN request member states to implement prevention, training and information measures intended for healthcare professionals and the general public.What does ANSM do?
- ANSM forwards national psychoactive substance evaluation reports produced by the CEIP-A centres to the WHO (World Health Organization) Expert Committee on Drug Dependence.
- ANSM participates in the United Nations Commission on narcotic drugs. It can propose or support resolutions.
SINTES (National Identification System for Toxic and Substances)
This system is a component of the TREND program (the French system for continuous monitoring of recent trends and new drugs established) by the OFDT (French Observatory for Drugs and Addictive Trends).
SINTES aims to enhance the understanding of the toxicological content of illicit drugs through:
SINTES aims to enhance the understanding of the toxicological content of illicit drugs through:
- An observation component (synthesizing toxicological analysis data of seized products and conducting specific collections from users);
- A reviewing exper;
- Website
Haemovigilance
Haemovigilance consists of monitoring, assessing and preventing incidents and adverse reactions, occurring in donors and recipients of labile blood products. This monitoring applies to the entire transfusion chain, i.e. from labile blood product collection to recipient follow-up.Haemovigilance monitors and assess:
- adverse reactions occurring in blood donors,
- post-donation information, liable to compromise the quality or safety of the blood products obtained from these donations or from previous donations,
- incidents in the transfusion chain, from Labile Blood Products collection to recipient follow-up,
- adverse reactions occurring in Labile Blood Products recipients.
The objective of transfusion safety is to identify hazards that have caused, cause or are liable to cause incidents or adverse reactions that have endangered, endanger, or may endanger the health of donors or recipients with a view to eliminating or reducing the associated risks.
The haemovigilance system is based on local, regional and national tiers.
ANSM sets out the main priorities and the objectives of the haemovigilance system, and manages the missions of the various parties involved. In some cases, it may refer to competent authorities.
- ANSM receives reports from the various parties involved. It is also kept informed of the appearance of obstacles to the quality and safety of the stages of the transfusion chain. In addition, it receives data from epidemiological blood donor monitoring.
- ANSM can also carry out epidemiological surveys with the French National Blood Service, the Army Blood Transfusion Centre, and the National Reference Centre for HIV and hepatitis B and C viruses in blood transfusion of the French National Blood Transfusion Institute.
These epidemiological surveys enable the observation and analysis of health problems in the population, and the determination of their causes and risk factors. - ANSM can conduct studies, for example studies pertaining to the conditions of use of labile blood products, within the framework of the haemovigilance network tasked with forwarding data relating to the health safety of blood products.
Events and incidents reporting and assessment
All healthcare professionals (nurse practitioner, doctor, pharmacist, midwife, dental surgeon, etc.) who observe or are aware of an adverse event in recipient, serious adverse events in donors, serious incident, post-donation information, should report it without delay to the haemovigilance and transfusion safety correspondents (in french CHV-ST) of the healthcare institution or blood transfusion clinic where the incident occurred or the adverse reaction was observed.The CHV-ST reports in the e-FIT national electronic haemovigilance reporting system. The registered report can be accessed in real time by any of the parties involved in the haemovigilance network concerned by this report, particularly CRH-ST coordinators and ANSM.
The report is reviewed on-the-fly by the relevant CRH-ST reference center for haemovigilance and transfusion safety (in french CHR-ST) and ANSM. It is discussed throughout its lifecycle among the haemovigilance and transfusion safety correspondents, CRH-ST coordinator and ANSM until the end of the investigations, with a view to determining in particular the attributability of the LBP(s) in question in the onset of the reported adverse reaction, in the light of the data already known, the context of use and the profile of the patient in question.
Find out more about French haemovigilance
Blood transfusion clinic correspondents
Must report the following to ANSM without delay:
Must report the following to ANSM:
Must report the following to the relevant correspondent without delay:
Local tier of the haemovigilance system
On the local tier, three categories of parties are involved in the haemovigilance system:Blood transfusion clinic correspondents
Must report the following to ANSM without delay:
- all adverse reactions occurring in an labile blood products recipient,
- all serious adverse reactions occurring in a blood donor,
- all serious transfusion chain incidents,
- all post-blood donation information.
Must report the following to ANSM:
- all adverse reactions occurring in a labile blood product recipient,
- all serious incidents.
Must report the following to the relevant correspondent without delay:
- all adverse reactions occurring in a labile blood product recipient,
- all serious adverse reactions occurring in a blood donor,
- all serious incidents,
- all post-blood donation information.
Regional tier of the haemovigilance system
The regional haemovigilance and transfusion safety coordinators (CRH-ST) work with the Director of the regional health authority and ensure the application of:- haemovigilance-specific rules and instructions,
- ANSM decisions,
- actions undertaken by health institutions tasked with haemovigilance and transfusion safety. They also monitor the implementation of haemovigilance, and the implementation of preventive and corrective measures proposed by health institutions and blood transfusion clinics.
Find out more about European and international haemovigilance
ANSM plays an active role in the European working groups with vigilance competence, in particular in the Vigilance Expert Subgroup, which track developments in national haemovigilance systems and identify signals of interest for the EU.
It is regularly requested in this context for surveys in the area of haemovigilance, and more broadly in the field of transfusion.
It participates in the RAB blood alert platform.
France’s contribution to European haemovigilance
- European Commission (EC) : European Member States (MS) report signals to the Commission, allowing them to be shared with the other Member States via a European blood alert platform, RAB (Rapid Alert Blood), and the annual reports of each MS.
- EC Vigilance Expert Subgroup on Substances of Human Origin : noteworthy events are discussed in this subgroup with the Council of Europe (CoE) with a view to sharing potential corrective actions of the EC with Member States
ANSM plays an active role in the European working groups with vigilance competence, in particular in the Vigilance Expert Subgroup, which track developments in national haemovigilance systems and identify signals of interest for the EU.
It is regularly requested in this context for surveys in the area of haemovigilance, and more broadly in the field of transfusion.
It participates in the RAB blood alert platform.
France’s contribution to international haemovigilance
- World Health Organization (WHO)
- International Haemovigilance Network (IHN)
- International Society of Blood Transfusion (ISBT)
Medical device vigilance
Medical device vigilance assesses incidents and risks of incidents involving a medical device. It is performed from the clinical trial phase, and throughout their life cycle.The medical device vigilance system is structured in three tiers. The national is managed by ANSM. The local tier involves local medical device vigilance correspondents who work in public or private healthcare institutions, healthcare professionals and manufacturers. They are all required to report any incidents or risks of incidents that come to their attention to ANSM. Additionally, there is a regional tier on a trial basis consisting of regional medical device vigilance correspondents.
This monitoring may result in preventive and corrective measures being adopted to prevent serious incidents or risks of serious incidents from occurring/recurring.
Find out more about French medical device vigilance
Data collection and recording by ANSM
This data is forwarded by:- local healthcare institution medical device vigilance correspondents,
- manufacturers,
- industry operators,
- users and third parties.
- serious incidents or risks of serious incidents,
- corrective actions or medical device market recalls implemented by manufacturers.
Incident assessment by ANSM
Four types of incidents are assessed.- Minor incidents: these are incidents of low severity. The manufacturer is not obliged to provide additional information.
- Major incidents: in this case, the manufacturer is obliged to carry out an investigation, which may be accompanied by an assessment of the device, conducted by the manufacturer or by a third party.
- Critical incidents: these incidents are the subject of an immediate assessment, and may be accompanied by a health measure. Additional information must be collected from the manufacturer and the reporting party.
- Comprehensive assessment: the assessed incidents are known, occur frequently, and may be serious. They involve a specific type of device. They are collected and are the subject of a statistical analysis.
Information and alerts issued by ANSM
There are three different types of information and alerts:- information or guidelines (issued by ANSM and intended for healthcare institutions or community pharmacists),
- corrective actions or recalls (approved by ANSM and issued by the manufacturer, intended for healthcare institutions or users),
- health policy decisions (adopted by ANSM and intended for manufacturers and other market operators: distributors, agents, etc.).
Reagent vigilance
Reagent vigilance assesses incidents and risks of incidents related to the use of in vitro diagnostic medical devices (IVDMDs).The reagent device vigilance system is also structured in three tiers. The national tier managed by ANSM. The local tier involves local local reagent device vigilance correspondents work in public or private healthcare institutions, healthcare professionals and manufacturers, who are required to report any incidents or risks of incidents that come to their attention to ANSM. Additionally, there is a regional tier on a trial basis, consisting of regional reagent device vigilance correspondents.
This monitoring may result in preventive and corrective measures being adopted to prevent serious incidents or risks of serious incidents from occurring/recurring.
Find out more about French reagent vigilance
Assessment of incidents and risks of incidents
Four types of incidents are assessed.- Minor incidents: these are incidents of low severity. The manufacturer is not obliged to provide additional information.
- Major incidents: in this case, the manufacturer is obliged to carry out an investigation, which may be accompanied by an assessment of the device, conducted by the manufacturer or by a third party.
- Critical incidents: these incidents are the subject of an immediate assessment, and may be accompanied by a health measure. Additional information must be collected from the manufacturer and the reporting party.
- Comprehensive assessment: the assessed incidents are known, occur frequently, and may be serious. They involve a specific type of device. They are collected and are the subject of a statistical analysis.
Information and alerts issued by ANSM
ANSM, working in collaboration with manufacturers, sends local reagent vigilance correspondents, laboratory managers, and also heads of institutions, and users two types of letters:- Letters relating to information and guidelines,
- Letters relating to recalls.
- Production, import, marketing suspensions,
- Bans on activities that have given rise to incidents,
- Application of restrictions or special conditions of use in respect of medical devices